Multiple Choice
An electron is in the n = 3 energy level in a hydrogen atom. To ionize this atom, it is necessary for the electron to gain a minimum of how much energy? See Figure 4-11. 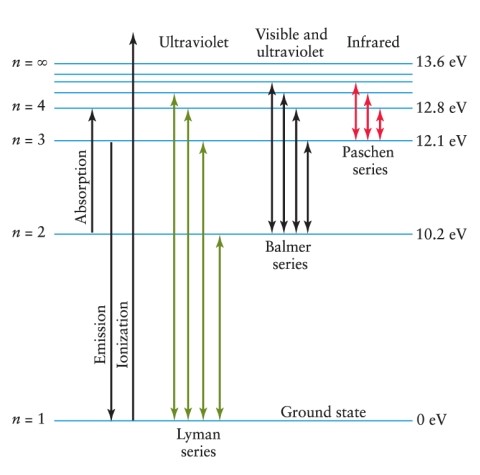
Definitions:
Related Questions
Q10: The carbon isotope <sup>14</sup>C is not useful
Q22: When visible light passes through a prism
Q30: The MOST likely mechanism for the formation
Q34: What is a neutron?<br>A) uncharged atom<br>B) uncharged
Q88: Which of these provides a plausible method
Q95: Earth rotates eastward, dragging eastward the tidal
Q95: The atomic number of the isotope <sup>238</sup>U
Q104: Which of these is the BEST explanation
Q121: How many electrons orbit the nucleus of
Q209: In the 1860s, Maxwell derived a set