An electron is in the n = 3 energy level in a hydrogen atom. To ionize this atom, it is necessary for the electron to gain a minimum of how much energy? See Figure 4-11. 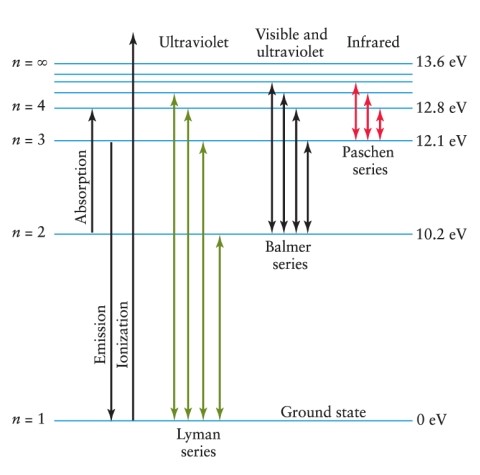
Definitions:
Startup Cost
Initial expenses incurred during the process of creating a new business, including but not limited to legal fees, marketing, and inventory costs.
Capital Structure
The composition of a company's liabilities and equity, including debt and equity financing, used to finance the company's operations and growth.
Debt and Equity
The composition of a company's capital, consisting of debt (loans and bonds) and equity (stocks), used for financing its operations.
New Project
A project that involves starting a new task, product, service, or process, which has not been undertaken by the organization before.
Q13: Which planet in our solar system has
Q21: The mass of the solar nebula that
Q30: What is the angle between the line
Q32: To date, all the exoplanets detected through
Q41: What is the reason for the fact
Q41: A Kuiper belt object whose orbit is
Q91: What happens to MOST of the neutrinos
Q96: Iron has 26 protons, and one isotope
Q127: Carbon-14 (<sup>14</sup>C) has a half-life of approximately
Q311: The development of the scientific theory related