What is wrong with the Lewis structure shown for sulfur trioxide, SO3? 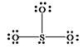
Definitions:
Teenage Unemployment
The scenario where individuals, typically aged 13-19, are actively seeking but unable to find work.
Economic Model
A simplified representation of economic processes, often using mathematical formulas or graphs, to analyze behavior and predict outcomes.
Inductive Reasoning
A method of reasoning in which a conclusion is reached based on the observation of specific instances or patterns.
Variable Theory
A conceptual framework that outlines how variables interact within a particular study or model.
Q1: Which of the following elements has the
Q7: Which of the following represents an advantage
Q19: The main buffer system found in blood
Q45: Which of the following is an essential
Q49: Which of the following accounts for the
Q53: If an atom gains one electron, it
Q64: How many moles of KCl are present
Q70: The atomic number of an ion indicates
Q81: Because iodine tends to concentrate in one
Q89: Because the C-H bond in methane is