What is wrong with the Lewis structure shown for sulfur trioxide, SO3? 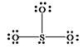
Definitions:
Sociobiologists
Scientists who study the biological aspects of social behavior in animals and humans, emphasizing genetics and evolutionary explanations.
Essentialists
Individuals or theorists who believe in essentialism, the idea that certain categories of people have intrinsically fixed, fundamental characteristics.
Genes
The basic physical and functional units of heredity, made up of DNA, that influence the development and functioning of living organisms.
Essentialist Perspective
The essentialist perspective is a belief that certain phenomena or categorizations are naturally occurring and immutable, often ignoring the role of social and cultural constructs.
Q1: Morphine is used medicinally as a pain
Q3: Distinguish between a type I error rate
Q12: According to the VSEPR theory, what is
Q18: How many total valence electrons are in
Q24: A balloon containing helium at constant pressure
Q25: A collision between reactant molecules that produces
Q31: The Aufbau Principle specifies which of the
Q66: The ionic compound magnesium hydroxide can be
Q72: Glycerol has a lower viscosity than ethanol.<br>
Q85: The law of conservation of mass states