What reactive intermediates are involved in the following reaction? 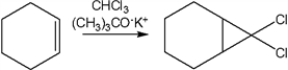
Definitions:
Polarity
A property of molecules having distinct ends with opposite charges, leading to unequal sharing of electrons.
Methane Molecule
A simple hydrocarbon with the chemical formula CH4, consisting of one carbon atom bonded to four hydrogen atoms, and is the primary component of natural gas.
Polarity
A property of molecules with distinct oppositely charged ends, resulting from an uneven distribution of electrons.
Solvents
Substances that dissolve a solute, resulting in a solution; usually liquid but can be gas or solid.
Q4: Which of the following is not true
Q12: Sample is bombarded with high-energy electrons<br>A)EI-MS<br>B)FAB<br>C)MALDI<br>D)ESI-MS
Q13: How could you distinguish between 1-butanol and
Q14: Which of the following combinations of peaks
Q21: What is the IUPAC name of the
Q22: Which of the following laws relates absorbance
Q29: Substance A is formed from the reaction
Q41: Which of the following energy diagrams represents
Q73: What is the best choice of reagent(s)
Q85: Which one of the following ketones cannot