Which of the following best represents an sp2 hybridized atomic orbital of carbon which overlaps with the 1s atomic orbital of hydrogen to form a C−H σ bonding molecular orbital in ethene, H2C=CH2 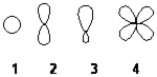
Definitions:
Merchandise Purchased
The total cost of goods bought for resale during a specific accounting period.
Purchases Discounts
A reduction in the price of goods that a buyer can take advantage of if they pay by a certain date.
Periodic Inventory System
An inventory method where updates to inventory levels are made at specified periods, typically at the end of a financial year, rather than continuously.
Periodic Inventory System
An inventory accounting method where inventory levels and cost of goods sold are calculated at set intervals, such as monthly or yearly.
Q1: Which actions would best aid the new
Q5: What is the physical,emotional,and spiritual exhaustion that
Q10: The 125-lb nurse is preparing to lift
Q13: The nurse avoids dragging the patient across
Q31: What is the index of hydrogen deficiency
Q37: Using the EZ system, the following would
Q37: The nurse is aware that the characteristic
Q55: What is the hybridization of carbon atoms
Q59: Which of the following molecular formulae corresponds
Q114: What is the ground-state electronic configuration of