Which of the following best represents an sp2 hybridized atomic orbital of carbon which overlaps with the 1s atomic orbital of hydrogen to form a C−H σ bonding molecular orbital in ethene, H2C=CH2 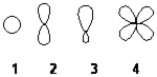
Definitions:
Mutualism
A type of symbiotic relationship between two species where both partners benefit from the interaction.
Predation
One species captures, kills, and eats another.
Parasitism
Relationship in which one species withdraws nutrients from another species, without immediately killing it.
Species Richness
Of a community, the number of species.
Q3: An employee failed to perform the duties
Q22: Which diagnostic test for diabetes mellitus provides
Q28: A mixture containing 0.50 mmol of each
Q29: What is the cranial nerve that supplies
Q31: The nurse knows which of the following
Q45: What is the smallest trans cycloalkene that
Q48: The following Newman projection represents 2-methylhexane. <img
Q77: How many isomers of 1,3,5-heptatriene exist?<br>A) 1<br>B)
Q116: What is the molecular formula of aspartame,
Q118: Which of the following is a