What is the shape around each carbon atom in the structure shown below? 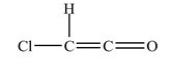
Definitions:
Exothermic
Describes a chemical reaction that releases energy in the form of heat.
Transition States
High-energy states through which reactants must pass for a chemical reaction to occur, representing a maximum in energy on the reaction path between reactants and products.
Endothermic
A reaction or process that absorbs heat from its surroundings, resulting in a temperature decrease in the external environment.
Frequency Factor
A term in chemical kinetics that represents the frequency of collisions that lead to a reaction, influenced by the orientation and energy of the reactants.
Q7: In the acid-base reaction: F<sup>-</sup>(aq)+ HNO<sub>3</sub>(aq) <img
Q18: A liquid solution that contains a nonvolatile
Q55: Which salt forms a solution with a
Q57: Which solution has the highest boiling point?<br>A)A
Q58: What is the structure of methoxycyclobutane?<br>A) <img
Q65: London dispersion forces are exhibited by all
Q70: Many organic compounds contain the carbonyl group
Q90: Ethambutol (structure shown)is a bacteriostatic antimycobacterial drug
Q97: How many quaternary carbons are in this
Q101: Fill in the missing product in the