Propanol,CH3CH2CH2OH,has the structure shown below. What is the strongest type of intermolecular force that exists between two propanol molecules? 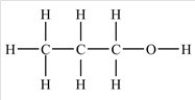
Definitions:
FTC Commissioners
Officials appointed to the Federal Trade Commission, responsible for enforcing antitrust and consumer protection laws in the United States.
Appeal Decision
is challenging a decision made by a lower court or administrative body, seeking a review and reversal by a higher court.
Consent Order
A consent order is a legal document in which parties in a dispute agree to a resolution without admitting guilt or wrongdoing, often used in regulatory matters and family law disputes.
Disputed Behavior
Refers to actions or conduct that are contended or contested by one or more parties in a legal or formal setting.
Q9: Consider the oxidation of sodium metal to
Q16: Vitamin B6,shown below,is a fat-soluble vitamin. <img
Q17: Which nuclear equation is an example of
Q29: Which type of Medical Imaging utilizes radioactivity?<br>A)X-Rays<br>B)CT
Q33: In the reaction: Ni<sup>2+</sup>(aq)+ Mg(s)→ Ni(s)+ Mg<sup>2+</sup>(aq),the
Q35: A scuba diver typically begins a dive
Q50: C<sub>2</sub>H<sub>6</sub> has a higher boiling point than
Q51: The (II)in the name of the ionic
Q63: To multiply two numbers in scientific notation,multiply
Q71: A mole is a quantity that contains