When octane evaporates,what kind of attractive forces are overcome? 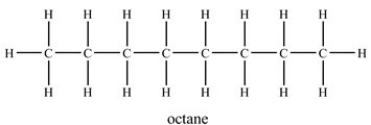
Definitions:
Recrystalization
Recrystallization is a metamorphic process where crystals of minerals grow larger while the overall chemical makeup of the rock remains the same, usually as a result of heat and/or pressure.
Remobilization
The process by which sedimentary deposits are eroded and transported to be deposited again in a new location.
Pressure Solution
A diagenetic process where mineral grains partially dissolve under high stress, typically in wet conditions, allowing deformation.
Regional Metamorphism
The process of transformation of rocks by heat and pressure over wide areas of the Earth's crust, typically associated with mountain building.
Q5: An alpha particle is identical to a
Q10: In order for a solution to conduct
Q13: Every atom must have an octet of
Q21: In calculating the percent yield,both the actual
Q22: The condensed structure and skeletal structure shown
Q24: A peanut butter and jelly sandwich contains
Q30: When forming an ion,an atom with the
Q58: What is the correct formula for the
Q65: London dispersion forces are exhibited by all
Q84: Atoms with seven valence electrons typically form