The molecular art depicts the following reversible reaction at equilibrium: CO(g) + Cl2(g)  COCl2(g) . What can be inferred about the equilibrium constant,K,for this reaction?
COCl2(g) . What can be inferred about the equilibrium constant,K,for this reaction? 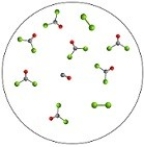
Definitions:
State Laws
The collection of laws and regulations enacted by individual states, governing various aspects of life and business within their respective jurisdictions.
Federal Laws
Statutes enacted by the national government that apply throughout the country, governing areas of legal jurisdiction not covered by state law.
Culture Of Inclusion
An organizational ethos that values diversity and promotes the inclusion of different perspectives, backgrounds, and talents.
Diversity Initiatives
Programs and policies designed to promote and embrace diversity within an organization, often focusing on inclusion across various dimensions such as race, gender, age, sexual orientation, and disability.
Q12: Write a balanced chemical equation for the
Q29: Consider the combustion reaction of propane: C<sub>3</sub>H<sub>8</sub>(g)+
Q29: Given these vapor pressures at 20 °C:
Q31: Energy is released when a less organized
Q46: Surface tension measures which of the following?<br>A)A
Q60: The melting point of a solution prepared
Q73: The process of solvent molecules surrounding solute
Q77: In the balanced redox reaction: 2 Cu(s)+
Q86: Which statement describing atoms is FALSE?<br>A)The number
Q97: Assume that the mixture of substances in