Consider the reversible reaction at equilibrium: N2(g) + O2(g) 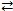 2 NO(g) . What is the effect of removing some N2(g) from the equilibrium system?
2 NO(g) . What is the effect of removing some N2(g) from the equilibrium system?
Definitions:
Differentiated Product
A good or service that is distinct from its competitors through variation in design, function, or quality.
Business Cycle
The fluctuations in economic activity that an economy experiences over a period of time, characterized by phases of growth (expansion) and decline (recession).
Game Theory
The study of how people behave in strategic situations in which individuals must take into account not only their own possible actions but also the possible reactions of others. Originally developed to analyze the best ways to play games like poker and chess.
Economies Of Scale
Cost advantages that enterprises obtain due to their scale of operation, typically associated with efficiencies in production as output increases.
Q14: Oxygen,carbon,hydrogen,and nitrogen are called the building-block elements
Q29: Given these vapor pressures at 20 °C:
Q40: C-H bonds are considered to be nonpolar,because
Q41: What is the period and Group for
Q49: _ is the passage of water and
Q56: In organic compounds,any atom that is not
Q62: A patient's systolic pressure is measured as
Q65: London dispersion forces are exhibited by all
Q66: The balanced reaction: 4 NO<sub>2</sub> + O<sub>2</sub>
Q94: Since living cells are surrounded by biological