Consider the following reversible reaction at equilibrium: CO(g)+ Cl2(g) 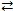 COCl2(g). If chlorine gas is added to the reaction vessel,the equilibrium will shift to the right.
COCl2(g). If chlorine gas is added to the reaction vessel,the equilibrium will shift to the right.
Definitions:
Chemical Hazards
Pertains to any chemical substance that can cause harm to individuals or the environment when improperly managed, stored, or used.
Biotechnology
The development of techniques to genetically manipulate organisms.
Fusion Reaction
A nuclear reaction where two light atomic nuclei combine to form a heavier nucleus, releasing energy in the process.
Atomic Weapons
Weapons that derive their destructive force from nuclear reactions, either fission or a combination of fission and fusion.
Q4: Nonmetals have a shiny appearance,and they are
Q18: Consider the balanced reaction: Zn(s)+ 2 HCl(aq)→
Q20: Phosphorus usually forms two covalent bonds in
Q54: Rank the atoms Br,Cl,and K in order
Q55: Which atom has the smallest atomic radius?<br>A)K<br>B)Ga<br>C)Br<br>D)Rb
Q70: Aspartic acid is an amino acid used
Q71: Which of the following statements concerning a
Q74: One would need to add _ calories
Q75: A catalytic converter uses a catalyst to
Q81: What is the density of a sample