The equilibrium constant expression for the reversible reaction: CHCl3(g)+ 3 HCl(g)  CH4(g)+ 3 Cl2(g)is
CH4(g)+ 3 Cl2(g)is 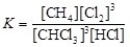 .
.
Definitions:
Borrow
The act of receiving something with the intent to return it, often referring to money in the context of loans where interest may be charged.
Standard Deviations
A statistic that measures the dispersion of a dataset relative to its mean, used to quantify the amount of variation or spread of a set of data values.
Stocks A and B
Placeholder names used in discussions to refer to two specific, but unspecified, stocks or to compare aspects of different stocks.
Q38: Hydrogen is located in group 1A but
Q40: Pure water has an osmotic pressure of
Q45: A student runs the reaction: LiOH +
Q47: Which statement is not part of the
Q57: Ethylene glycol is expected to be less
Q58: What is the molar mass of the
Q62: A patient's systolic pressure is measured as
Q71: An atom of the isotope chlorine-37 consists
Q71: The principal buffer in the blood is
Q85: The mass of a neutron is equal