Multiple Choice
Consider the reversible reaction at equilibrium: N2(g) + O2(g) 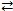 2 NO(g) . What is the effect of removing some N2(g) from the equilibrium system?
2 NO(g) . What is the effect of removing some N2(g) from the equilibrium system?
Definitions:
Related Questions
Q20: Which bonding pattern is NOT typical of
Q30: Which species is the conjugate acid of
Q35: What is the pH of a buffer
Q57: What is the formula for the ionic
Q66: Which molecule is more water soluble?<br>A) <img
Q67: The reaction of a Brønsted-Lowry acid (HA)with
Q72: The formula for dinitrogen pentoxide is N<sub>5</sub>O<sub>2</sub>.
Q90: Red Blood Cells are placed into a
Q101: Fill in the missing product in the
Q117: In order to complete the structure below,_