Which statement concerning the reversible reaction 2 NO2(g) 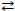 N2O4(g) is true?
N2O4(g) is true?
Definitions:
Periodic Amounts
Regularly scheduled payments or receipts over a defined period.
Interest
The cost of borrowing money or the return on investment for savings and investments, typically expressed as an annual percentage rate.
Liquidity Ratios
Financial metrics used to assess a company's ability to meet its short-term debt obligations, by comparing current assets to current liabilities.
Debt-Paying Ability
A financial metric used to gauge a firm's capacity to settle its obligations, often assessed through ratios such as the debt to equity or debt service coverage ratios.
Q20: Phosphorus usually forms two covalent bonds in
Q33: Liquid solutions are always transparent and colorless.
Q45: Neutral atoms always contain an equal number
Q46: How many total valence electrons does the
Q49: Combustion reactions are redox reactions.
Q62: A patient's systolic pressure is measured as
Q71: When the volume of a sample of
Q71: An atom of the isotope chlorine-37 consists
Q73: Which unit of radioactivity is equivalent to
Q95: Which element is a d block element?<br>A)S<br>B)Ar<br>C)Ag<br>D)As<br>E)None