Consider the following reversible reaction at equilibrium: CO(g)+ Cl2(g) 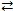 COCl2(g). If chlorine gas is added to the reaction vessel,the equilibrium will shift to the right.
COCl2(g). If chlorine gas is added to the reaction vessel,the equilibrium will shift to the right.
Definitions:
Alpha Proteobacteria
A class of Proteobacteria that includes a variety of species, some of which are capable of photosynthesis or nitrogen fixation.
Gram-Negative Bacteria
Bacteria with cell walls consisting of a thin peptidoglycan layer and a thick outer membrane. Compare with gram-positive bacteria
Cyanobacteria
Photosynthetic bacteria that produce oxygen, found in aquatic and terrestrial environments, sometimes causing blooms.
Photosynthesis
The process by which green plants, algae, and some bacteria convert light energy into chemical energy in the form of glucose, using carbon dioxide and water.
Q3: Which is NOT an ionic compound?<br>A)KCl<br>B)Ca(NO<sub>3</sub>)<sub>2</sub><br>C)CBr<sub>4</sub><br>D)AlBr<sub>3</sub>
Q9: An endothermic reaction is one in which
Q12: Write a balanced chemical equation for the
Q14: What is the molarity of a solution
Q27: Which species completes the nuclear equation shown
Q48: Which gas law describes the relationship between
Q49: How many lone pairs of electrons need
Q82: A strong acid readily donates a proton,forming
Q83: Aspartic acid is an amino acid used
Q93: An aqueous solution of KCl has a