Consider the following reversible reaction at equilibrium: CO(g)+ Cl2(g) 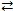 COCl2(g). If the pressure inside the reaction vessel is increased,the equilibrium will shift to the left.
COCl2(g). If the pressure inside the reaction vessel is increased,the equilibrium will shift to the left.
Definitions:
Marginalize
To relegate or confine to a lower or outer edge of society, often limiting access to resources and opportunities for those deemed outside the mainstream.
Language
A systematic means of communicating by the use of sound or conventional symbols, used for human communication through the expression of thought and emotion.
Sapir-Whorf Hypothesis
A theory in linguistics that suggests the structure of a language influences its speakers' world view or cognition.
Social Reality
The layers of reality perceived through social constructs and interactions.
Q12: When the liquid carbon tetrachloride (density =
Q33: The Lewis structure shown below is not
Q34: Which is the correct Lewis structure for
Q37: The subscripts in chemical formulas are changed
Q40: Complete the following nuclear fission equation: <img
Q64: Consider the reaction: 2 C<sub>2</sub>H<sub>6</sub>(g)+ 7 O<sub>2</sub>(g)→
Q68: What is the electron configuration of an
Q70: A solution is made by dissolving 3.88
Q81: The molecules A and B react according
Q87: Once equilibrium is reached in a chemical