Consider the following reversible reaction at equilibrium: C6H12O6 (aq)+ 6 O2(g) 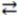 6 CO2(g)+ 6 H2O(l). If carbon dioxide gas is removed from the reaction vessel,the equilibrium will shift to the right.
6 CO2(g)+ 6 H2O(l). If carbon dioxide gas is removed from the reaction vessel,the equilibrium will shift to the right.
Definitions:
Inpatient Hospital
A hospital setting where patients are admitted and stay overnight or longer, receiving medical treatment and care.
Physician's Office
A medical facility where doctors and other healthcare professionals provide consultations, diagnoses, and treatment to patients.
Urgent Care
Healthcare services provided for illnesses or injuries that require prompt attention but are not serious enough to warrant an emergency room visit.
ICD-10-CM
The International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) is a system used in the United States to classify and code diagnoses, symptoms, and procedures recorded in conjunction with hospital care.
Q2: How many valence electrons are in a
Q10: A solution containing a low concentration HCl
Q11: Which species completes the nuclear equation shown
Q14: Which of the statements concerning chemical bonds
Q38: Hydrogen is located in group 1A but
Q55: Consider the following reversible reaction at equilibrium:
Q63: The sulfite ion contains more oxygen atoms
Q69: Freeze-drying removes water from foods by the
Q71: The electron configuration of the oxide ion
Q99: Which acid has the strongest conjugate base?<br>A)Hydrogen