Consider the reversible reaction: CO(g)+ Cl2(g) 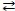 COCl2(g). The reverse reaction is COCl2(g)→ CO(g)+ Cl2(g).
COCl2(g). The reverse reaction is COCl2(g)→ CO(g)+ Cl2(g).
Definitions:
Dishonest Answers
Responses provided by individuals that intentionally hide the truth or mislead, often for personal gain or to avoid negative outcomes.
Correlation
Usually a number between +1.0 and -1.0 that indicates whether and how much two variables are related. Correlation indicates whether an increase in one variable will increase or decrease another variable. Correlation indicates only that two variables are somehow related, not that one variable causes the other to increase or decrease.
Variables
Elements or factors that can change and affect the outcome of experiments or studies, often manipulated or measured by researchers.
Causal Link
The relationship between two events where one is the result of the occurrence of the other; a cause-effect relationship.
Q2: Which K value below is consistent with
Q11: Isotopes of the same element have the
Q11: The molecular shape around the boron atom
Q37: Iodine has smaller ionization energy than chlorine.
Q53: Consider the reaction: PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7327/.jpg"
Q63: What are the products of the acid-base
Q64: Consider the reaction: 2 C<sub>2</sub>H<sub>6</sub>(g)+ 7 O<sub>2</sub>(g)→
Q87: The farther a shell is from the
Q87: To decrease the incidence of harmful bacteria
Q98: A chemical reaction requires 31.39 kJ. How