Consider the mixture of Cl2 and F2 in a closed container as illustrated below. What will the contents of the container look like if the molecules undergo the reaction: Cl2(g) + 3 F2(g) → 2 ClF3(g) ? 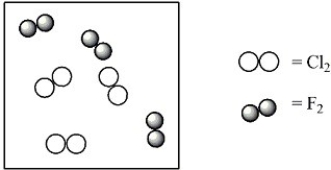
Definitions:
Q3: Considering the electronegativity values indicated for each
Q12: When the liquid carbon tetrachloride (density =
Q57: Ethylene glycol is expected to be less
Q58: What is the correct formula for the
Q59: Which molecule or ion has ONLY two
Q68: The mass of one ethanol (C<sub>2</sub>H<sub>6</sub>O)molecule is
Q75: What is the atomic number of the
Q81: Hydrogen-3 is a radioactive isotope of hydrogen.
Q83: The Ca<sup>2+</sup> ion contains _ protons.<br>A)20<br>B)18<br>C)22<br>D)40<br>E)2
Q89: In the acid-base reaction: HCN(aq)+ F<sup>-</sup>(aq) <img