True/False
The structures shown below are resonance structures of sulfur dioxide. 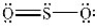 and
and 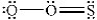
Definitions:
Related Questions
Q4: The solubility of helium gas in water
Q7: How many protons are in the isotope
Q16: A chemical equation is an expression that
Q30: When using learning stations for interactive family
Q36: Two solutions with the same osmotic pressure
Q36: Potassium sulfide has the chemical formula K<sub>2</sub>S.
Q42: Catalysts accelerate a reaction by _.<br>A)lowering the
Q63: The sulfite ion contains more oxygen atoms
Q85: In a chemical reaction,the actual yield of
Q88: What is the molarity of a 25.0%