The following figure illustrates the action of a HF and F- buffer where the sizes of the boxes are proportional to the concentrations of HF and F- in solution.What will happen when a small amount of base (OH-) is added to the HF/F- buffer? 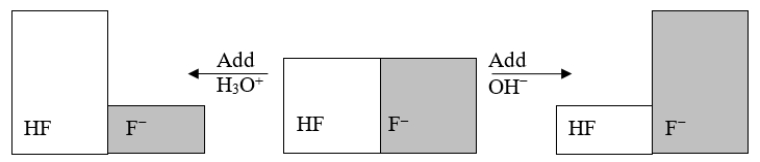
Definitions:
Japanese Colonialism
The period of history when Japan expanded its empire by annexing territories in Asia and the Pacific, from the late 19th century to the mid-20th century.
Transportation Networks
Systems comprised of roads, railways, airports, and waterways that facilitate the movement of people and goods.
Hydroelectric Power
Renewable energy generated by using the movement of water through dams to produce electricity.
Japanese Colonialism
The period during which Japan extended its influence and control over various territories and peoples, primarily in Asia, through colonization, expansion, and imperial administration.
Q5: Firearms amnesty programs have been found to
Q7: Discuss the importance of police acts,policing standards,and
Q9: The practice of police officers accepting favourable
Q21: Research on the use of force by
Q24: Which buffer system is the primary buffering
Q38: Which of the following are conjugate acid-base
Q54: A nitrogen bubble with a volume of
Q71: You want to create a table based
Q77: Jane Doe has a cholesterol (C<sub>27</sub>H<sub>46</sub>O)count of
Q79: The neutralization reaction of potassium hydrogen carbonate