What is the strongest type of intermolecular force of attraction that a caffeine molecule could form with water? 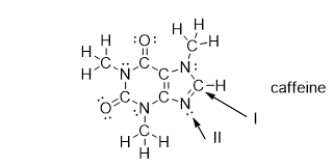
Definitions:
Paid In Full
A term indicating that a debt or obligation has been completely settled and no balance remains due.
Uncorrected
Refers to something that has not been amended, fixed, or rectified.
Single Line
A straight line that has no bends or curves.
Numeric Amount
A figure or value expressed in numbers, representing quantity or magnitude.
Q34: In addition to single bonds, which of
Q37: All acid-base reactions that we consider in
Q45: Which of the following atomic diagrams best
Q56: What is the mass number of an
Q63: Which solute is MOST soluble in hexane?<br>A)
Q71: Which choice BEST describes the purpose of
Q72: The boxed species in the following reaction
Q72: Metabolism is<br>A) the breakdown of food.<br>B) the
Q73: Which statement about DNA transcription is TRUE?<br>A)
Q85: Which of the following reactions releases the