The figure shows the energy levels for an electron in a finite potential energy well.If an electron in the n = 2 state absorbs a photon of wavelength 2.0 nm, what happens to the electron? 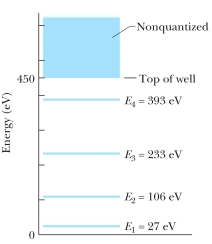
Definitions:
Similar Needs
Refers to the common or comparable requirements and desires of individuals or market segments.
Q24: Which molecule has nonpolar covalent bonds?<br>A) NO<br>B)
Q28: A typical mammogram X-ray results in a
Q32: The length of a meter stick moving
Q33: The Diels-Alder reaction is very important in
Q36: The preferred conformation of cis-3-tert-butyl-1-methylcyclohexane is the
Q38: Light from a small region of an
Q43: The critical angle for total internal
Q47: When a lithium atom is made from
Q51: Light of uniform intensity shines perpendicularly on
Q71: Two of Maxwell's equations contain a path