The potential energy for the interaction between the two atoms in a diatomic molecule is U = A/x12 - B/x6, where A and B are constants and x is the interatomic distance.The magnitude of the force that one atom exerts on the other is: 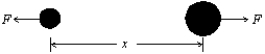
Definitions:
Pressure Flow Theory
A model explaining how sugars are transported in plants, suggesting that sugars move from sources, where they are produced, to sinks, where they are used or stored, through the phloem via pressure gradients.
Nitrogen-Fixing Bacteria
Bacteria that convert atmospheric nitrogen into ammonia, a form accessible to plants for nutrition.
Essential Elements
Nutrients required for organisms to perform vital bodily functions and sustain life, which cannot be synthesized by the organism.
Cohesion-Tension Theory
A theory that explains the movement of water and nutrients from roots to leaves in plants through the cohesive and adhesive properties of water.
Q1: A vector in the xy plane
Q16: Block A, with a mass of 4.0
Q22: Mars has a mass of about 0.1075
Q37: A uniform 240-g meter stick can be
Q43: Given the perihelion distance, aphelion distance, and
Q55: A 1100-kg elevator is rising and its
Q56: The coordinate of a particle in meters
Q58: A 2.0-kg block starts from rest on
Q64: A thin-walled hollow tube rolls without sliding
Q82: A wheel initially has an angular velocity