The two panels in the figure below display the same data.Explain why the trends look different between the two graphs. 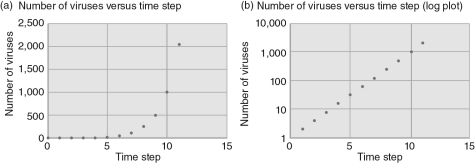
Definitions:
Alpha Decay
A type of radioactive decay in which an atomic nucleus emits an alpha particle (two protons and two neutrons bound together) and transforms into a different element.
Atomic Nucleus
The atomic nucleus is the small, dense region at the center of an atom, composed of protons and neutrons, and is the core where most of an atom's mass is concentrated.
Ionizing Power
The ability of a chemical element or electromagnetic radiation to ionize atoms or molecules by altering their charge through the removal or addition of electrons.
Beta Particles
High-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei during radioactive decay.
Q5: A large region of _ wavelengths are
Q8: Scientific notation is used in astronomy primarily
Q16: Newton's first law states that an object
Q18: In the figure below, the person on
Q35: Photographic plates provide an improvement over naked-eye
Q50: In the figure below, which blackbody spectrum
Q68: The wavelength of a wave is<br>A) the
Q74: In science an idea that cannot be
Q81: Explain the difference between dispersion and diffraction.How
Q103: Natural abilities that people possess are called:<br>A)interests.<br>B)aptitudes.<br>C)attitudes.<br>D)personality