The difference in energy between the n = 2 and n =1 electronic energy levels in the hydrogen atom is 1.6 *10-18 J.If an electron moves from the n = 1 level to the n=2 level, will a photon be emitted or absorbed? What will its energy be, and what type of electromagnetic radiation is it? Use the electromagnetic spectrum shown in the figure below to answer this question. 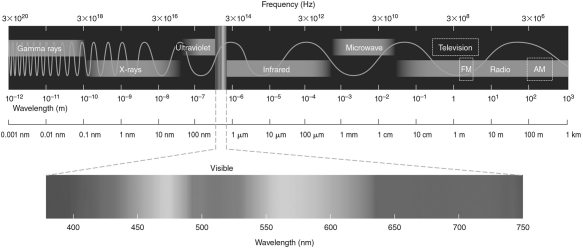
Definitions:
New York
A state in the northeastern U.S., known for its cultural, financial, and media capital, New York City, which encompasses iconic sites like the Statue of Liberty, Empire State Building, and Central Park.
Lynching
An extrajudicial killing by a group, primarily used in the United States to describe the murder of African Americans by mob action.
Black Women's Clubs
Organizations formed in the late 19th and early 20th centuries by African American women to address issues of civil rights, education, and welfare in their communities.
Segregation
describes the enforced separation of different racial, ethnic, or social groups within a society.
Q3: Assume you are observing the night
Q14: Which molecule moves with the fastest average
Q15: A(n) _ is a location on Earth
Q17: What are some advantages and disadvantages of
Q34: Large-scale winds are generated on Earth primarily
Q40: Why do reflecting telescopes use curved mirrors
Q41: If the flux of sunlight on a
Q61: Six months from now, at what time
Q64: Galileo's telescopic observations of the _ led
Q91: Typically, video is shot using 24-30 frames