The difference in energy between the n = 2 and n =1 electronic energy levels in the hydrogen atom is 1.6 *10-18 J.If an electron moves from the n = 1 level to the n=2 level, will a photon be emitted or absorbed? What will its energy be, and what type of electromagnetic radiation is it? Use the electromagnetic spectrum shown in the figure below to answer this question. 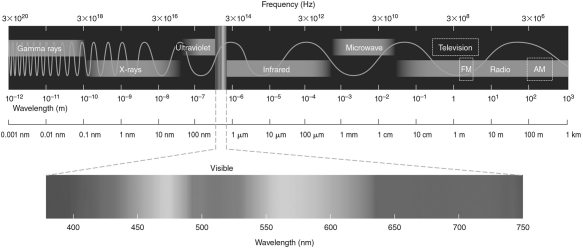
Definitions:
Net Method
An accounting approach that records transactions after deducting any discounts, rather than recording them at their gross amount and including the discounts as a separate item.
Purchase Discounts
Price reductions given by suppliers to buyers as an incentive for early payment of their purchase invoices.
Journalize
The process of recording business transactions in the journal, making note of the debit and credit of each transaction as per double-entry bookkeeping rules.
Inventory Valuation
The process of determining the monetary value of a company's inventory, using methods such as FIFO (First-In, First-Out), LIFO (Last-In, First-Out), and weighted average cost.
Q2: The majority of the nitrogen in the
Q26: Chromatic aberration results from<br>A) blue light being
Q27: The force of gravity between Earth and
Q49: The telescope was invented by<br>A) Galileo Galilei,
Q54: The primary atmosphere of Earth consisted of
Q61: Based on the figure below, a superior
Q64: Astronomers believe that the "hot Jupiters" found
Q70: Why does the Moon always show the
Q92: What is the difference between visible light
Q96: How does the fraction of oxygen in