A hydrogen atom makes a downward transition from the 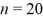 state to the n = 5 state. Find the wavelength of the emitted photon. The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J, c = 3.00 × 108 m/s)
state to the n = 5 state. Find the wavelength of the emitted photon. The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J, c = 3.00 × 108 m/s)
Definitions:
Investing Activities
Transactions involving the acquisition and disposal of long-term assets and other investments not included in cash equivalents.
Valuable Assets
Resources or possessions of an entity that are considered to hold significant value or importance.
Accounting
The systematic process of recording, analyzing, summarizing, and reporting financial transactions of a business or individual.
Financial Information
Refers to data encompassing a company's financial status, including earnings, expenses, debts, and investments.
Q2: The energy of a proton is 1.0
Q2: When introducing an exercise, leaders should clearly
Q5: If the directions are not _in the
Q9: How far are you from your image
Q10: An electron is bound in an infinite
Q18: To become an effective group leader, these
Q23: A tube with a 3.0-mm radius has
Q27: Intervening between two members who are talking
Q32: The effective leader of a counseling or
Q43: The radioactivity due to carbon-14 measured in