What is the change in entropy of 10.0 moles of ideal monatomic gas that reversibly undergoes the isothermal expansion shown in the figure? The ideal gas constant is R = 8.314 J/(mol ∙ K) . 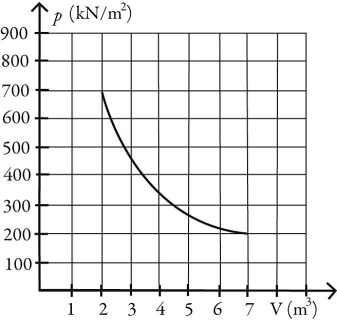
Definitions:
Protective Strategy
A method or approach designed to safeguard or defend against potential threats, dangers, or undesirable outcomes.
Denial
A defense mechanism in which confrontation with a personal problem or with reality is avoided by denying the existence of the problem or reality.
Repression
A defense mechanism where undesirable thoughts, feelings, or urges are unconsciously pushed out of conscious awareness.
Super-Ego
In Freudian psychology, the part of the mind that acts as a self-critical conscience, reflecting social standards learned from parents and teachers.
Q1: A fixed amount of ideal gas is
Q6: The figure shows three electric charges labeled
Q10: A cylinder contains 23 moles of an
Q14: A bag of potato chips contains 2.00
Q27: At a certain depth in the ocean,
Q27: A nonuniform electric field is directed along
Q40: A 15-μF air-filled capacitor is connected to
Q55: What is the maximum length of a
Q57: A particle with charge -5.00 C initially
Q60: The temperature of an ideal gas in