A heat engine takes 2.0 moles of an ideal gas through the reversible cycle abca, on the pV diagram shown in the figure. The path bc is an isothermal process. The temperature at c is 820 K, and the volumes at a and c are 0.010 m3 and 0.16 m3, respectively. The molar heat capacity at constant volume, of the gas, is 37 J/mol ∙ K, and the ideal gas constant is R = 8.314 J/(mol ∙ K) . The thermal efficiency of the engine is closest to 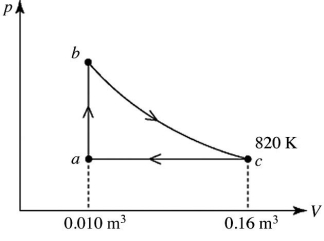
Definitions:
Mood
A psychological state that can affect perception, behavior, and physical well-being.
Autonomy
The capacity and right of individuals to make their own choices and decisions, free from coercion or external influence.
Moral Principles
Fundamental beliefs about what is right and wrong or good and bad behavior.
Beneficence
The principle of doing good and preventing harm, often discussed as an ethical foundation in medicine and healthcare.
Q5: A conducting sphere contains positive charge distributed
Q9: A 25-L container holds ideal hydrogen (H<sub>2</sub>)
Q14: The root-mean-square speed (thermal speed) of the
Q22: The cross section of a long coaxial
Q23: An object drops a distance h in
Q24: Charge Q<sub>1</sub> = 6.0 nC is at
Q34: A sealed 89- <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7476/.jpg" alt="A sealed
Q41: On planet X, the absolute pressure at
Q78: A cube at 100.0°C radiates heat at
Q82: Heat is added to a pure substance