What is the change in entropy of 10.0 moles of ideal monatomic gas that reversibly undergoes the isothermal expansion shown in the figure? The ideal gas constant is R = 8.314 J/(mol ∙ K) . 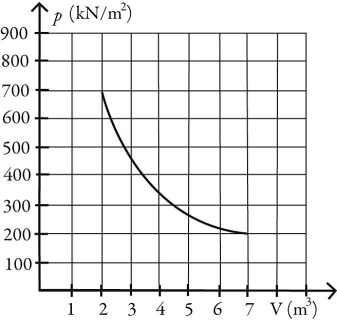
Definitions:
External Party
An individual or organization that is outside the primary organizations or individuals involved in a particular situation or transaction.
Contract Bid
The process of submitting a proposal to undertake a specific project or job, often within a competitive context.
Strict Guidelines
Rigorous and precise instructions or rules that must be followed carefully.
Sales Proposal
A written document designed to offer products or services to potential buyers, outlining the benefits, pricing, and terms of the sale to persuade the customer to make a purchase.
Q2: Incompressible water flows out of a large
Q2: Two concentric conducting spherical shells produce a
Q6: A light bulb is connected to a
Q25: A satellite is in circular orbit at
Q33: The compressor in a certain Carnot refrigerator
Q37: An ideal gas is kept in a
Q40: You are driving a late model convertible
Q46: A 2.0-m string is fixed at both
Q48: Water flowing through a pipe suddenly comes
Q69: A wire segment 1.2 m long carries