A 810-g quantity of ethanol, in the liquid state at its melting point of 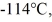 is frozen at atmospheric pressure. The heat of fusion of ethanol is 1.04 × 105 J/kg, the molecular mass is 46.1 g/mol, and the ideal gas constant is R = 8.314 J/(mol ∙ K) . The change in the entropy of the ethanol as it freezes is closest to
is frozen at atmospheric pressure. The heat of fusion of ethanol is 1.04 × 105 J/kg, the molecular mass is 46.1 g/mol, and the ideal gas constant is R = 8.314 J/(mol ∙ K) . The change in the entropy of the ethanol as it freezes is closest to
Definitions:
Selling Office Furniture
The act of disposing or trading office furniture, typically to acquire newer items, manage space, or liquidate assets.
Industry Averages
Statistical metrics that represent the average performance of companies within a particular industry, useful for benchmarking.
Financial Data
Quantitative details about an entity's financial position, performance, and changes in financial position that users rely on to make economic decisions.
Analytical Measures
Techniques used to understand, interpret, and improve business performance through statistical and quantitative analysis.
Q3: The graph in the figure shows the
Q9: A ten-loop coil having an area of
Q11: A 0.28-kg block on a horizontal frictionless
Q13: A radiating body originally has a Kelvin
Q20: The moons of Mars, Phobos (Fear) and
Q28: A neutral hollow spherical conducting shell of
Q47: As you stand by the side of
Q57: Consider the circuit shown in the figure.
Q67: One of the dangers of tornados and
Q84: In a thermodynamic process involving 7.8 moles