What is the average translational kinetic energy per molecule of an ideal gas at a temperature of 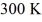 ? The Boltzmann constant is 1.38 × 10-23 J/K.
? The Boltzmann constant is 1.38 × 10-23 J/K.
Definitions:
Drinking Behaviors
Patterns of alcohol consumption that can range from moderate and responsible to excessive and harmful.
Contextual
Pertaining or relating to the circumstances that form the setting for an event, statement, or idea, which are considered as affecting its meaning or significance.
Relational
Pertaining to or concerning the way in which two or more concepts, objects, or people are connected.
Purpose Of Assessment
The rationale or objective behind evaluating or measuring a particular set of skills, knowledge, abilities or performance.
Q3: A solid nonconducting sphere of radius R
Q5: In an LC circuit containing a 40-mH
Q31: The figure shows a pV diagram for
Q37: Two in-phase loudspeakers are some distance apart.
Q42: An isolated air-filled parallel-plate capacitor that is
Q53: Five capacitors are connected across a potential
Q59: A bar magnet is held vertically with
Q69: An ideal gas is allowed to expand
Q71: During an adiabatic process, 20 moles of
Q79: A solenoid with 400 turns has a