Assuming the radius of diatomic molecules is approximately 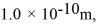 for what pressure will the mean free path in room-temperature (20°C) nitrogen be 4.6 m? The Boltzmann constant is 1.38 × 10-23 J/K, Avogadro's number is 6.02 × 1023 molecules/mole, and the ideal gas constant is
for what pressure will the mean free path in room-temperature (20°C) nitrogen be 4.6 m? The Boltzmann constant is 1.38 × 10-23 J/K, Avogadro's number is 6.02 × 1023 molecules/mole, and the ideal gas constant is 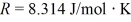 = 0.0821 L ∙ atm/mol ∙ K.
= 0.0821 L ∙ atm/mol ∙ K.
Definitions:
Confidential Business Data
Information proprietary to a business that is kept secret to maintain an advantage or prevent it from being exploited by competitors.
Branding
The marketing practice of creating a name, symbol, or design that identifies and distinguishes a product from other products.
Container
An object or space used to hold, store, or carry items, ranging from small boxes to large shipping containers.
Catchy Phrase
A memorable and appealing sentence or slogan often used in advertising to attract attention.
Q7: A parallel-plate capacitor has plates of area
Q9: Two concentric spheres are shown in the
Q16: Two identical loudspeakers that are 5.00 m
Q18: As shown in the figure, a rectangular
Q18: You are driving along a highway at
Q21: An aluminum wire and a steel wire,
Q26: Betelgeuse is a red supergiant star in
Q30: A sound source emits 20.0 W of
Q31: Two thin 80.0-cm rods are oriented at
Q40: For the circuit shown in the figure,