Assuming the radius of diatomic molecules is approximately 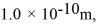 for what pressure will the mean free path in room-temperature (20°C) nitrogen be 4.6 m? The Boltzmann constant is 1.38 × 10-23 J/K, Avogadro's number is 6.02 × 1023 molecules/mole, and the ideal gas constant is
for what pressure will the mean free path in room-temperature (20°C) nitrogen be 4.6 m? The Boltzmann constant is 1.38 × 10-23 J/K, Avogadro's number is 6.02 × 1023 molecules/mole, and the ideal gas constant is 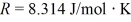 = 0.0821 L ∙ atm/mol ∙ K.
= 0.0821 L ∙ atm/mol ∙ K.
Definitions:
Corporate Global Strategy
A business approach that defines a company's goals, tactics, and policies to compete and operate internationally.
Labor Relations
The study and practice of managing the relationships between employers and employees, often focusing on collective bargaining, unions, and workers' rights.
Corporate Internationalization
The expansion of a company's operations and influence beyond its national borders, entering foreign markets through trade, investment, or partnerships.
Unions
Organizations formed by workers to protect their rights and interests, often engaging in collective bargaining with employers.
Q12: A 0.10- <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7476/.jpg" alt="A 0.10-
Q13: A hollow conducting spherical shell has radii
Q19: Four traveling waves are described by the
Q22: An LR circuit contains an ideal 60-V
Q24: Electric charge is uniformly distributed inside a
Q25: The exterior of a supersonic airplane is
Q25: In the figure, a ring 0.71 m
Q59: An ideal gas with γ = 1.67
Q71: Consider a solenoid of length L, N
Q79: A solid uniform sphere of mass 1.85