How many grams of ice at -13°C must be added to 711 grams of water that is initially at a temperature of 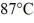 to produce water at a final temperature of
to produce water at a final temperature of 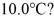 Assume that no heat is lost to the surroundings and that the container has negligible mass. The specific heat of liquid water is 4190 J/kg ∙ °C and of ice is 2050 J/kg · °C. For water the normal melting point is 0.00°C and the heat of fusion is 334 × 103 J/kg. The normal boiling point is 100°C and the heat of vaporization is 2.26 × 106 J/kg.
Assume that no heat is lost to the surroundings and that the container has negligible mass. The specific heat of liquid water is 4190 J/kg ∙ °C and of ice is 2050 J/kg · °C. For water the normal melting point is 0.00°C and the heat of fusion is 334 × 103 J/kg. The normal boiling point is 100°C and the heat of vaporization is 2.26 × 106 J/kg.
Definitions:
Historical Significance
Describes the importance or impact that an event, person, or place has had on history.
Labor Movement
A social and economic campaign to improve the rights, working conditions, and compensation of workers.
Social Darwinism
An ideology that applies the concept of "survival of the fittest" to human societies, justifying inequalities in wealth and social status as natural.
Liberty of Contract
A legal principle asserting the freedom of individuals to form contracts without government interference, emphasizing economic freedom and limiting state regulation.
Q6: Calculate the current through a 10.0-m long
Q7: The figure shows two unequal point charges,
Q16: As shown in the figure, two long
Q23: In the figure Q = 5.8 nC
Q23: The pV diagram shown is for 7.50
Q35: Two thin-walled concentric conducting spheres of radii
Q39: A certain metal has a coefficient of
Q45: A frictionless simple pendulum on Earth has
Q46: If we double only the spring constant
Q95: In an isochoric process, the internal (thermal)