Multiple Choice
The temperature of an ideal gas in a sealed 0.40- 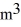 rigid container is reduced from 350 K to
rigid container is reduced from 350 K to 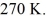 The final pressure of the gas is
The final pressure of the gas is 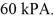 The molar heat capacity at constant volume of the gas is 28.0 J/mol · K. The heat absorbed by the gas is closest to
The molar heat capacity at constant volume of the gas is 28.0 J/mol · K. The heat absorbed by the gas is closest to
Understand the principles behind optimal choice and utility maximization.
Apply knowledge of microeconomics to analyze real-world consumer behavior scenarios.
Understand the types of feedback suitable for on-the-spot interventions in social work.
Grasp the concept and application of structural mapping in modifying family alignments.
Definitions:
Related Questions
Q7: A solid sphere, solid cylinder, and a
Q8: The cross section of a long coaxial
Q12: The hole for a bolt in a
Q21: Which statements are true for an electron
Q22: Four capacitors are connected across a 90-V
Q24: Ekapluto is an unknown planet that has
Q32: Two identical resistors of resistance R =
Q38: A system consists of two very large
Q65: A copper sphere has a radius of
Q90: A uniform solid sphere has a moment