An expansion process on an ideal diatomic gas has a linear path between the initial and final states on a pV diagram. The initial pressure is  the initial volume is
the initial volume is 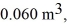 and the initial temperature is
and the initial temperature is 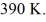 The ideal gas constant is R = 8.314 J/mol ∙ K. The final pressure is
The ideal gas constant is R = 8.314 J/mol ∙ K. The final pressure is 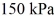 and the final temperature is
and the final temperature is 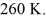 The work done by the gas is closest to
The work done by the gas is closest to
Definitions:
Q5: For a fixed amount of gas, if
Q13: A 12-L volume of oil is subjected
Q14: Two automobiles traveling at right angles to
Q29: Two equal point charges Q are separated
Q32: The graph in the figure shows a
Q38: If the electric flux through a closed
Q40: For the circuit shown in the figure,
Q47: Tensile stress is<br>A) the strain per unit
Q51: A rigid rectangular loop, which measures 0.30
Q81: If two forces of equal magnitude act