A fixed amount of ideal gas goes through a process abc. In state a, the temperature of the gas is 152°C, its pressure is 1.25 atm, and it occupies a volume of 0.250 m3. It then undergoes an isothermal expansion to state b that doubles its volume, followed by an isobaric compression back to its original volume at state c. (Hint: First show this process on a pV diagram.) The ideal gas constant is 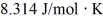 , and 1.00 atm = 1.01 × 105 Pa.
, and 1.00 atm = 1.01 × 105 Pa.
(a) How many moles does this gas contain?
(b) What is the change in the internal energy of the gas between states a and b?
(c) What is the net work done on (or by) this gas during the entire process?
(d) What is the temperature of the gas in state c?
Definitions:
Simple Rate
An interest calculation method where interest is not compounded on earned interest, instead applied only on the principal amount.
Principal Plus Interest
It is the total amount due that includes the original amount borrowed (principal) in addition to the charged interest on that principal.
Cheque Accounting
The handling and record-keeping of cheques within the accounting practices of an individual or organization.
Interest Earning
Relates to the income generated from investing or depositing funds in interest-bearing financial instruments.
Q1: A fixed amount of ideal gas is
Q5: A non-conducting sphere of radius R =
Q13: A uniform meter stick is freely pivoted
Q18: As shown in the figure, a rectangular
Q26: A galvanometer coil having a resistance of
Q26: A 5.0-liter gas tank holds 1.7 moles
Q28: The figure shows two connected wires that
Q40: Ocean tides are waves that have a
Q40: A 1.0-kg block and a 2.0-kg block
Q45: A 6.0-m wire with a mass of