A 648-g empty iron kettle is put on a stove. How much heat. in joules. must it absorb to raise its temperature from 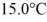 to
to 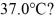 (The specific heat for iron is 113 cal/kg ∙ C°, 1 cal = 4.190 J)
(The specific heat for iron is 113 cal/kg ∙ C°, 1 cal = 4.190 J)
Definitions:
Dependent Demand
Demand for components or materials directly tied to the production of another product, typically calculated rather than forecasted.
Quick Response
An inventory strategy that aims to reduce lead times and increase supply chain flexibility to meet customer demand more effectively.
Multiple Orders
The process of placing or receiving more than one order for products or services at the same time or within a similar timeframe.
Manufacturer
An entity or business that produces goods, usually on a large scale, involving machinery, tools, and labor.
Q4: Two people are talking at a distance
Q6: A nonconducting spherical shell of inner radius
Q8: The figure shows two arcs of a
Q12: A heavy stone of mass m is
Q19: A multiloop circuit is shown in the
Q32: A very long straight wire carries a
Q33: Consider a uniform solid sphere of radius
Q34: A charged particle is moving with speed
Q39: An object of mass 8.0 kg is
Q48: The gas in a perfectly insulated system