Multiple Choice
If you add 700 kJ of heat to 700 g of water at 70.0°C, how much water is left in the container? The latent heat of vaporization of water is 2.26 × 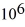 J/kg and its specific heat is 4190 J/(kg ∙ K) .
J/kg and its specific heat is 4190 J/(kg ∙ K) .
Definitions:
Related Questions
Q2: The power rating of a 400-Ω resistor
Q8: Three capacitors, with capacitances <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7476/.jpg" alt="Three
Q14: A 7.8-kg solid sphere, made of metal
Q16: The potential as a function of position
Q17: A frictionless pendulum clock on the surface
Q19: A blacksmith is flattening a steel plate
Q30: A sound source emits 20.0 W of
Q35: A lawn roller in the form of
Q38: A person is hearing two sound waves
Q51: A potter's wheel, with rotational inertia 46