The figure shows a pV diagram for 8.3 g of nitrogen gas (N2) in a sealed container. The temperature T1 of the gas in state 1 is 79°C. What are (a) the pressure p1 of the gas in state 1 and (b) the temperature T2 of the gas in state 2? The ideal gas constant is R = 8.314 J/mol ∙ K = 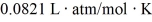 , and the ATOMIC weight of nitrogen is 14 g/mol.
, and the ATOMIC weight of nitrogen is 14 g/mol. 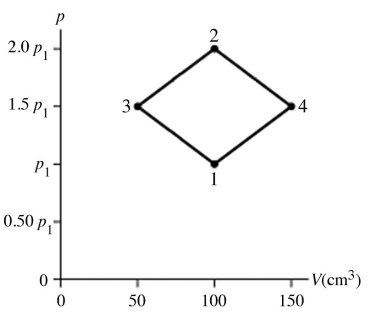
Definitions:
Shareholders
Individuals or entities that own shares in a corporation and have potential voting rights and the possibility of dividends.
Stakeholders
Individuals or groups that have an interest, financial or otherwise, in the performance and actions of an organization or project.
Bandwagon Fallacy
The misleading argument that something is true, good, or desired because many other people believe in it or do it.
Argumentum Ad Baculum
A logical fallacy in which an argument is made through appeal to force or threat instead of reason.
Q10: A conducting sphere is charged up such
Q11: During an isothermal process, 5.0 J of
Q15: A howler monkey is the loudest land
Q23: An object drops a distance h in
Q26: How many moles of water (H<sub>2</sub>O) molecules
Q27: The simple harmonic motion of an object
Q35: A lawn roller in the form of
Q69: The rotating systems shown in the figure
Q75: The filament in a light bulb has
Q84: A 5.0-m long, 12-kg uniform ladder rests