The figure shows a pV diagram for 4.3 g of oxygen gas (O2) in a sealed container. The temperature T1 of the gas in state 1 is 21°C. What are the temperatures T3 and T4 of the gas in states 3 and 4? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K, and the ATOMIC weight of oxygen is 16 g/mol. 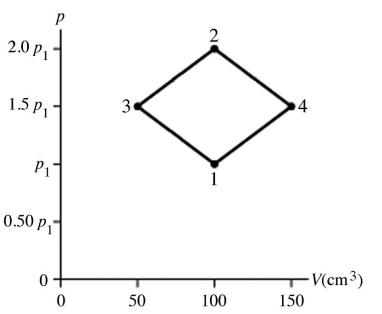
Definitions:
Investment Earnings
The return or income generated from various forms of investments, such as stocks, bonds, or real estate.
Present Value
A financial concept that calculates the current value of a future sum of money or stream of cash flows, given a specific rate of return.
Discount Rate
The interest rate used to discount future cash flows to their present value, important in investment and financial decision making.
Q2: A long, straight wire with <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7476/.jpg"
Q3: A half-ring (semicircle) of uniformly distributed charge
Q3: The graph in the figure shows the
Q5: What is the radius of a sphere
Q13: A hot air balloon has a volume
Q21: The capacitive network shown in the figure
Q36: An engine manufacturer makes the claim that
Q40: For the circuit shown in the figure,
Q80: A cube at 100°C radiates heat at
Q113: A child is trying to stack two