An expansion process on an ideal diatomic gas has a linear path between the initial and final states on a pV diagram. The initial pressure is 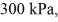 the initial volume is
the initial volume is 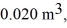 and the initial temperature is
and the initial temperature is 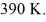 The final pressure is
The final pressure is 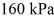 and the final temperature is
and the final temperature is 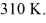 The change in the internal (thermal) energy of the gas is closest to
The change in the internal (thermal) energy of the gas is closest to
Definitions:
Q16: If an electron is accelerated from rest
Q28: An ideal gas is at a pressure
Q37: Two in-phase loudspeakers are some distance apart.
Q38: Consider two less-than-desirable options. In the first
Q40: When a vapor condenses,<br>A) the temperature of
Q48: In the figure, when the terminal voltage
Q55: A ball is released from rest on
Q59: A galvanometer has an internal resistance of
Q75: The filament in a light bulb has
Q79: A solenoid with 400 turns has a