A cylinder contains 1.2 moles of ideal gas, initially at a temperature of 116°C. The cylinder is provided with a frictionless piston, which maintains a constant pressure of 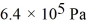 on the gas. The gas is cooled until its temperature has decreased to 27°C. For the gas
on the gas. The gas is cooled until its temperature has decreased to 27°C. For the gas
CV = 11.65 J/mol · K, and the ideal gas constant R = 8.314 J/mol ∙ K.
(a) Find the work done by (or on) the gas during this process. Is the work done by or on the gas?
(b) What is the change in the internal (thermal) energy of the gas during this process?
(c) How much heat is transferred to (or from) the gas during this process? Does this heat flow into or out of the gas?
Definitions:
Population Segments
Specific groups within a larger population differentiated by characteristics like age, gender, income, etc., targeted by businesses for marketing.
Latchkey Children
Refers to children who return to an empty home after school or are left unsupervised for long periods because their parents are at work.
Generation X
The demographic cohort following the Baby Boomers, typically defined as being born from the mid-1960s to early 1980s.
Generational Cohort
A group of people of the same generation—typically have similar purchase behaviors because they have shared experiences and are in the same stage of life.
Q1: Equal but opposite charges Q are placed
Q1: Two large, flat, horizontally oriented plates are
Q15: A solid uniform brick is placed on
Q17: Is it possible to transfer heat from
Q21: In a certain electroplating process gold is
Q22: A cubic box with sides of 20.0
Q30: An air-filled capacitor stores a potential energy
Q33: The compressor in a certain Carnot refrigerator
Q42: A coaxial cable consists of an inner
Q59: An ideal gas with γ = 1.67