A sealed 26- 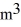 tank is filled with 2000 moles of oxygen gas (O2) at an initial temperature of 270 K. The gas is heated to a final temperature of 460 K. The ATOMIC mass of oxygen is 16.0 g/mol, and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K. The final pressure of the gas is closest to
tank is filled with 2000 moles of oxygen gas (O2) at an initial temperature of 270 K. The gas is heated to a final temperature of 460 K. The ATOMIC mass of oxygen is 16.0 g/mol, and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K. The final pressure of the gas is closest to
Definitions:
Productivity
A measure of the efficiency of a person, machine, factory, system, etc., in converting inputs into useful outputs.
Labour Hour
The unit of measure representing one hour of work performed by an employee, often used to track productivity and labor costs.
Signature Requirement
A stipulation that a document must be signed by an authorized party to validate its authenticity, enforceability, or to meet regulatory compliance.
Q2: A glass flask has a volume of
Q2: A time-varying horizontal force F(t) = At<sup>4</sup>
Q8: Waves travel along a 100-m length of
Q8: A person makes ice tea by adding
Q9: The voltage and power ratings of a
Q29: The process shown in the T-V diagram
Q31: A 2.3-kg object traveling at 6.1 m/s
Q31: Is it possible for a system to
Q51: A plane flies toward a stationary siren
Q76: In the figure, a weightlifter's barbell consists