The figure shows a pV diagram for 4.3 g of oxygen gas (O2) in a sealed container. The temperature T1 of the gas in state 1 is 21°C. What are the temperatures T3 and T4 of the gas in states 3 and 4? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K, and the ATOMIC weight of oxygen is 16 g/mol. 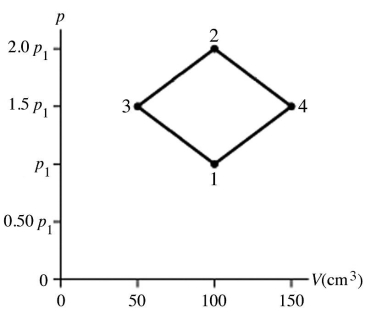
Definitions:
Cost of Funds
The interest rate that financial institutions pay for the use of money or funds they lend out to borrowers.
Principal
Principal refers to the original sum of money borrowed in a loan or invested, exclusive of any interest or dividends.
Annual Payments
Periodic payments made once every year, often used in terms of loans, insurance, and investments.
Outstanding Balance
The amount of money owed on a loan or credit line that has not yet been repaid.
Q2: The current supplied by a battery as
Q6: A 1.2-kg spring-activated toy bomb slides on
Q13: A certain crying baby emits sound with
Q18: A car heading north collides at an
Q21: The process shown in the pV diagram
Q34: The root-mean-square speed (thermal speed) of the
Q40: Ocean tides are waves that have a
Q52: A 50-cm<sup>3</sup> block of wood is floating
Q55: An adiabatic compression is performed on an
Q56: A 6.1-kg solid sphere, made of metal