Vector 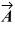 = -3.00
= -3.00  + 3.00
+ 3.00  and vector
and vector 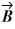 = 3.00
= 3.00  + 4.00
+ 4.00  . What is vector
. What is vector 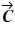 =
= 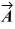 +
+ 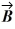 ?
?
Definitions:
Hydrocarbons
Organic compounds composed exclusively of hydrogen and carbon atoms, which can vary in structure, including alkanes, alkenes, and aromatics.
Saturated
Describes a molecule that contains no double or triple carbon-carbon bonds, with all carbon atoms connected by single bonds.
Alkane
A saturated hydrocarbon consisting of carbon and hydrogen atoms with single covalent bonds.
Secondary Carbons
A carbon atom bound to two other carbon atoms within an organic molecule, often influencing the molecule's reactivity and physical properties.
Q11: A dog is standing in the bed
Q12: If a country sets a pegged exchange
Q20: 0.00325 × 10<sup>-8</sup> cm can also be
Q25: The currency adopted by most countries in
Q28: A box of mass m is pulled
Q42: A child throws a ball with an
Q45: In the figure, four point masses are
Q65: The "Big Mac Theory of Exchange Rates"
Q80: The Danish currency, the krone, is pegged
Q138: When exchange rates are not determined in